DOWN TO 6 YEARS OLD
FOR MILD TO MODERATE ATOPIC DERMATITIS
ZORYVE® ITRELIEVE ITANYWHERE
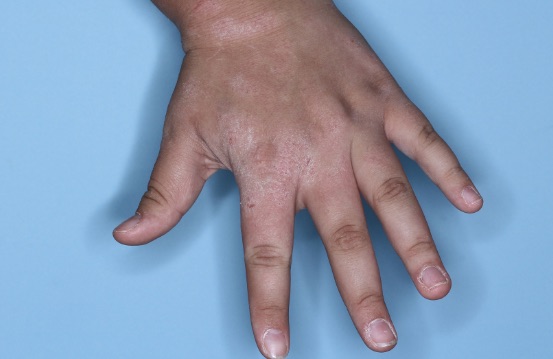
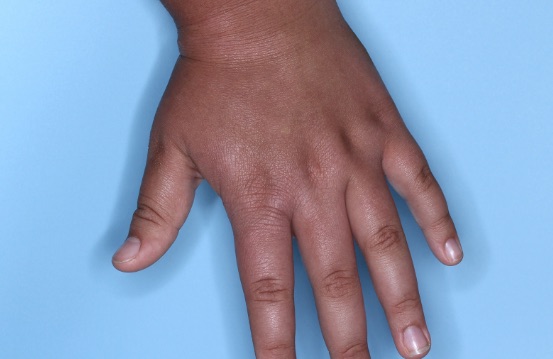
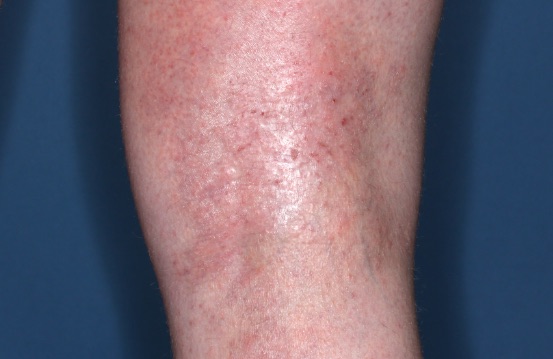
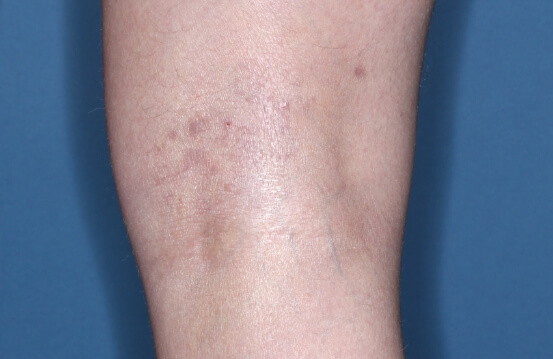
ZORYVE is for topical use only and not for ophthalmic, oral, or intravaginal use.1Symptoms illustrated. Not an actual patient.
ZORYVE has the power and versatility to be your everyday topical1
ZORYVE is for topical use only and not for ophthalmic, oral, or intravaginal use.1
SIMPLIFY ATOPIC DERMATITIS RELIEF WITH ZORYVE (zor-EEV)
31% of patients achieved vIGA-AD Success at Week 4 with ZORYVE vs 14% with vehicle, some as early as Week 1.2,3
32% of patients achieved WI-NRS Success at Week 4 with ZORYVE vs 17% with vehicle, with results observed within 24 hours.3
vIGA-AD = Validated Investigator Global Assessment-Atopic Dermatitis. vIGA-AD Success = Achievement of Clear (0)/ Almost Clear (1) and a ≥2-grade improvement from baseline. WI-NRS = Worst Itch Numeric Rating Scale. WI-NRS Success = ≥4-point improvement for patients with a baseline score ≥4. WI-NRS: 0 (no itch) to 10 (worst imaginable itch).
Committed to affordable patient access across all indications
One ZORYVE Direct Savings Program helps eligible, commercially insured patients get access and start ZORYVE treatment quickly and easily*†

Learn about patient
access support
*Prescriptions will be delivered to the patient 1–2 days after processing.
†Subject to eligibility criteria and maximum program limitation. This offer is not valid for patients without commercial drug insurance or whose prescription claims are eligible to be reimbursed, in whole or in part, by any government program. Please see Terms and Conditions.