
ITCH RELIEF
ITCH IMPROVEMENT AT WEEK 4 WITH RESULTS OBSERVED WITHIN 24 HOURS
Symptoms illustrated. Not an actual patient.
TWICE AS MANY PATIENTS ACHIEVED ITCH IMPROVEMENT AT WEEK 4 WITH ZORYVE3
2Xas many patients achieved WI-NRS Success at Week 4 with ZORYVE (32%; n=542) vs with vehicle (17%; n=271), P<0.0001, nominal3*
Secondary endpoint
Greater itch improvement observed within 24 HOURS after first application with ZORYVE as compared to vehicle3*†
*Data are pooled analyses of the INTEGUMENT 1 & 2 studies.
†Daily WI-NRS was a prespecified exploratory endpoint. Patients were asked to keep a daily diary logging itch symptoms and their severity. Day 1 represents baseline, first application. Evaluated in all patients, not just those with baseline WI-NRS score ≥4. Pooled analysis P-values are nominal.
WI-NRS = Worst Itch Numeric Rating Scale. WI-NRS Success =≥4-point improvement for patients with a baseline score ≥4. WI-NRS scale: 0 (no itch) to 10 (worst imaginable itch).
BASELINE
vIGA-AD = 3
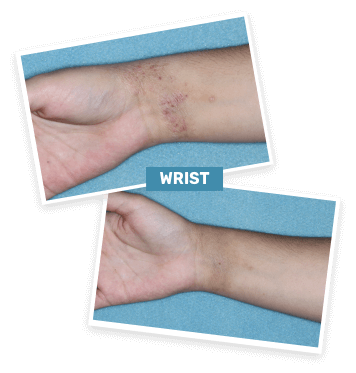
WEEK 4
vIGA-AD = 0
Actual clinical trial patient