
CLEARANCE RATES
CLEARER SKIN AS EARLY AS WEEK 1 FOR ATOPIC DERMATITIS
Actor portrayal
RAPID, RELIABLE RESULTS ANYWHERE
vIGA-AD Success
vIGA-AD
Clear/Almost Clear
Primary endpoint
vIGA-AD Success
at Week 4
2Xas many patients achieved vIGA-AD Success at Week 4 with ZORYVE vs vehicle (31% vs 14%)2,3
Significantly more patients
achieved vIGA-AD Success
as early as Week 12,3*
*Data are pooled analyses of the INTEGUMENT 1 & 2 studies.
vIGA-AD = Validated Investigator Global Assessment-Atopic Dermatitis. vIGA-AD Success = Achievement of a vIGA-AD Score of Clear (0)/ Almost Clear (1) and a ≥2-grade improvement from baseline.
Secondary endpoint
Clear/Almost Clear
at Week 4
Nearly2Xas many patients had Clear or Almost Clear skin at Week 4 with ZORYVE vs vehicle (41% vs 21%)2,3
Significantly more patients
achieved Clear/Almost Clear
skin as early as Week 12,3*
*Data are pooled analyses of the INTEGUMENT 1 & 2 studies.
vIGA-AD = Validated Investigator Global Assessment-Atopic Dermatitis.
BASELINE
vIGA-AD = 3
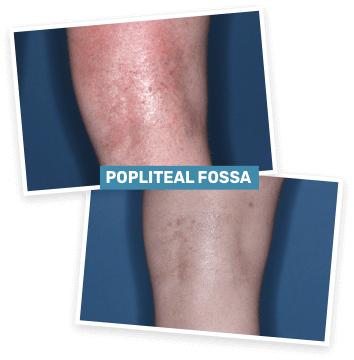
WEEK 4
vIGA-AD = 1
Actual clinical trial patient
9 in 10 patients
had improvements in clinical signs at Week 46
Most patients had improved EASI scores with ZORYVE monotherapy at Week 4
See itch relief data
Pooled EASI response rates of the INTEGUMENT 1 & 2 studies. Analysis provides a summary of EASI improvement for every patient in INTEGUMENT 1 & 2. EASI response rates are defined as percentage of patients with improved EASI scores from baseline to Week 4. EASI scores evaluate the following atopic dermatitis symptoms: erythema, skin thickness (induration, papulation, swelling), excoriations, lichenification, and percentage of region affected.
EASI = Eczema Area and Severity Index. EASI-50 = 50% reduction in EASI score from baseline. EASI-75 = 75% reduction in EASI score from baseline. EASI-90 = 90% reduction in EASI score from baseline. EASI-100 = 100% reduction in EASI score from baseline.
See itch relief data