
Actor portrayal

Actor portrayal
Study Design
STUDIED ACROSS MULTIPLE CLINICAL PRESENTATIONS
DERMIS-1 and DERMIS-2 study design:
Two Phase 3 randomized, parallel, double-blind, vehicle-controlled, multicenter studies
881 participants with plaque psoriasis – ZORYVE = 576, vehicle = 305
Once daily for 8 weeks
7% mean BSA involvement (range: 2%-20%)1,7
No concomitant therapies or other moisturizers/emollients were allowed on treated areas7
- Diagnosis of mild, moderate, or severe plaque psoriasis
- 2%–20% BSA
- Age ≥2 years (range 6–88)
Primary Endpoint
Key Secondary Endpoints
IGA Success at WEEK 8
Achievement of an IGA score of Clear (0)/ Almost Clear (1) and a ≥2-grade improvement from baseline
IGA = Investigator Global Assessment.
I-IGA Success at WEEK 8
Achievement of an I-IGA score of Clear (0)/ Almost Clear (1) and a ≥2-grade improvement from baseline
WI-NRS Success at WEEKS 8, 4, and 2
Achievement of a reduction of ≥ 4 points in subjects with a WI-NRS score of ≥ 4 at baseline
I-IGA = Intertriginous Investigator Global Assessment. WI-NRS = Worst Itch Numeric Rating Scale. WI-NRS: 0 (no itch) to 10 (worst imaginable itch).
DERMIS-1 and DERMIS-2 study participants reflected everyday patients
Participants presented with multiple affected body areas (N=881)7
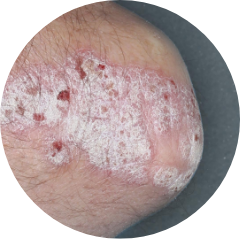
ELBOWS
72%
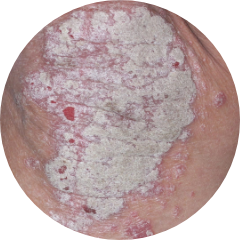
KNEES
58%
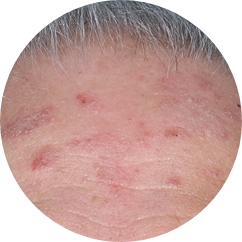
FACIAL
27%
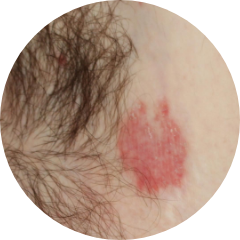
INTERTRIGINOUS
AREAS 21%
Actual clinical trial patients
Studied across all disease severities (IGA score at baseline)1
77% reported itch
with a baseline WI-NRS ≥4 (mean = 7.0)1,7
77% REPORTED ITCH with a baseline WI-NRS ≥4 (mean = 7.0)1,7
BSA = Body Surface Area. QD = Once daily. WI-NRS = Worst Itch Numeric Rating Scale.